Abstract
A body of pre-clinical evidence shows how the gut microbiota influence brain functioning, including brain connectivity. Linking measures of brain connectivity to the gut microbiota can provide important mechanistic insights into the bi-directional gut-brain communication. In this systematic review, we therefore synthesized the available literature assessing this association, evaluating the degree of consistency in microbiota-connectivity associations. Following the PRISMA guidelines, a PubMed search was conducted, including studies published up to September 1, 2022. We identified 16 studies that met the inclusion criteria. Several bacterial genera, including Prevotella, Bacteroides, Ruminococcus, Blautia, and Collinsella were most frequently reported in association with brain connectivity. Additionally, connectivity of the salience (specifically the insula and anterior cingulate cortex), default mode, and frontoparietal networks were most frequently associated with the gut microbiota, both in terms of microbial diversity and composition. There was no discernible pattern in the association between microbiota and brain connectivity. Altogether, based on our synthesis, there is evidence for an association between the gut microbiota and brain connectivity. However, many findings were poorly replicated across studies, and the specificity of the association is yet unclear. The current studies show substantial inter-study heterogeneity in methodology and reporting, limiting the robustness and reproducibility of the findings and emphasizing the need to harmonize methodological approaches. To enhance comparability and replicability, future research should focus on further standardizing processing pipelines and employing data-driven multivariate analysis strategies.
Similar content being viewed by others
Introduction
Neuronal connections are determined by the distance between neurons. Spatially closer neurons have a higher probability of being connected than those further away. As a result, the structural wiring of the brain represents a complex network with clusters of highly connected regions (i.e., structural connectivity) [1] that provide a basis for functional communication between brain areas (i.e., functional connectivity) as measured by the temporal coincidence of neuronal activation patterns of anatomically separated brain regions [2]. Patterns of communication between fixed sets of brain regions form functional networks [3, 4], which can be identified both during active cognitive tasks and during rest.
Functional networks measured during rest (resting-state networks) reflect, among others, processes related to cognitive functions. For example, the salience network (SN) is involved in the detection of behaviorally relevant stimuli [5], the frontoparietal network (FPN) is involved in the coordination of cognitive control [6], and the default mode network (DMN) is linked to basal, stimulus-independent cognitive processes such as information integration and mind-wandering [7]. In addition to cognitive functioning, connectivity may also reflect intrinsic processes, such as emotional and interoceptive awareness [8, 9]. Dysfunction in connectivity networks is observed in a range of psychiatric and neurodevelopmental disorders, including Attention Deficit/Hyperactivity Disorder [10] and Schizophrenia [11], in certain cases even prior to diagnosis [12].
The functional and structural connectivity patterns of the brain are affected by numerous genetic and non-genetic interacting factors. Our genetic makeup has a significant biological effect on both brain structure and function: heritability studies have shown that the additive genetic contribution explains approximately 50% to 93% of the variance in structural connectivity [13] and 20% to 40% of the variance in functional connectivity [14]. Likewise, the brain can change under the influence of environmental factors. Multiple studies have reported changes in connectivity strength following a mindfulness training, both in structural [15] and functional connectivity [16]. Additionally, a systematic review concluded that a lower quality diet was related to decreased structural and functional connectivity of default mode, sensorimotor and attention networks [17].
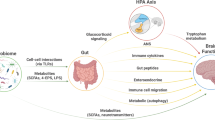
Such environmental factors, including diet, may exert their influence on the brain through the gut-brain axis (GBA), among others via modulation of the gut microbiota [18]. The GBA refers to the bidirectional communication system connecting the gastrointestinal system with the central nervous system (CNS) through endocrine, immune, and neural/vagal pathways [19]. The gut microbiota, comprising the trillions of microbes (predominantly bacteria) residing in the intestines, can modulate gut-brain communication, for example through the production of neuroactive metabolites, and by affecting the integrity of the gastro-intestinal and blood-brain barriers [19, 20].
A majority of the studies investigating the (microbiota-)gut-brain axis (MGBA) in humans focus on behavioral measures, including clinical diagnoses and questionnaires, providing evidence for a link between the gut microbiota composition and cognitive and emotional functioning [21,22,23]. In recent years the number of studies incorporating neuroimaging into the microbiota-gut-brain investigation has also increased rapidly. Specifically, the acquisition of functional and structural connectivity data is relatively standardized and often done at rest (i.e., without task instructions), making it a suitable research method for many participant populations, including children and patients. Linking such connectivity measures to the gut microbiota can provide important mechanistic insights into the bi-directional gut-brain communication. Therefore, we herein systematically review the available studies associating the gut microbiota with brain connectivity, in an effort to evaluate the degree of consistency in this association.
Method
Search strategy
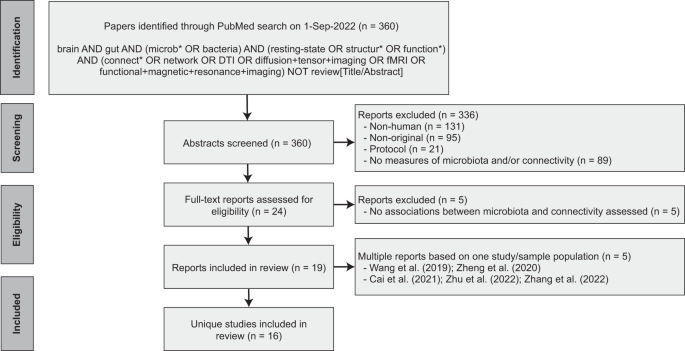
Following PRISMA guidelines, a systematic search on the PubMed database was conducted for reports published up to September 1, 2022. The aim was to capture all human studies that 1) collected a fecal sample to assess the gut microbiota, 2) assessed in vivo functional or structural brain connectivity, and 3) performed statistical analysis on the association between the gut microbiota and brain connectivity. Only peer-reviewed original research studies (i.e., reporting original data, analyses, and findings) published in English were included. Two independent raters (DM, MB) reviewed the titles and abstracts and came to a consensus about study inclusion. After inclusion, the following data was independently extracted by two authors (DM, MB): demographics, sample characteristics, method of gut microbiota estimation, method of brain connectivity estimation, statistical methods, and relevant results. Details on the search strategy and study inclusion are provided in Fig. 1. The PRISMA checklist is available in the Supplementary Materials.
PRISMA flow diagram detailing the database search, number of reports screened and number of studies included.
Consistency of the findings
The consistency of the findings was assessed on two levels. First, we looked at the gut microbiota and brain connectivity individually. Second, we assessed the specificity of the microbiota-connectivity association. For the first, the assessment was done by dividing the number of studies reporting a statistically significant association for a diversity index/genus abundance/brain network by the total number of studies assessing it. For the second, the assessment was done by counting the number of studies reporting a statistically significant microbiota-connectivity association and dividing it by the total number of studies that could have identified this association. Only findings assessed in at least three studies were interpreted. A finding was considered consistent if it was reported in at least 50% of the studies (if assessed in four or more studies), with an absolute minimum of two studies (if assessed in three studies).
Quality assessment
Two authors (DM, MB) assessed the risk of bias in the included studies using the National Institutes of Health (NIH) National Health, Lung and Blood Institute Study Quality Assessment Tool for observational Cohort and Cross-sectional Studies [24]. The tool was modified to also be suitable for case-control and before-after studies with no control group (specified in Supplementary Table 3). We used the method section of the STORMS checklist (v1.03) [25] to assess gut microbiota measurements, and an adaptation of the guidelines published by Poldrack et al. [26] to assess brain connectivity measurements (Supplementary Table 4-5). Study quality was rated as ‘Good’ for assessments of 75% or higher; ‘Fair’ for assessments between 50% and 75%; or ‘Poor’ for assessments lower than 50%.
Results
A comprehensive literature search on PubMed yielded 360 reports, of which a total of 19 publications based on 16 unique studies met the inclusion criteria (Fig. 1). Two of the included studies produced more than one publication ([27,28,29] and [30, 31]). For those studies, the findings reported in the individual publications were pooled.
Population characteristics (Table 1)
There was a wide variation in the target populations. Eight studies were conducted in healthy individuals [27,28,29, 32,33,34,35,36,37] including a study conducted in smokers [32], in newborns [36], and in infants [35]. The other eight studies were performed in a range of disease populations, including bipolar disorder [38], cirrhosis [39], end stage renal disease [30, 31], irritable bowel syndrome [40, 41], major depressive disorder [42], obesity [43] and patients undergoing a laparoscopic/vertical sleeve gastrectomy [44, 45]. Four of those studies were case-controlled [30, 31, 38, 41, 43] and three were longitudinal [40, 44, 45]. All other studies performed associations between gut microbiota composition and brain connectivity based on a single group and timepoint. Further characteristics of the study populations are listed in Table 1.
Methodological characteristics (Table 2)
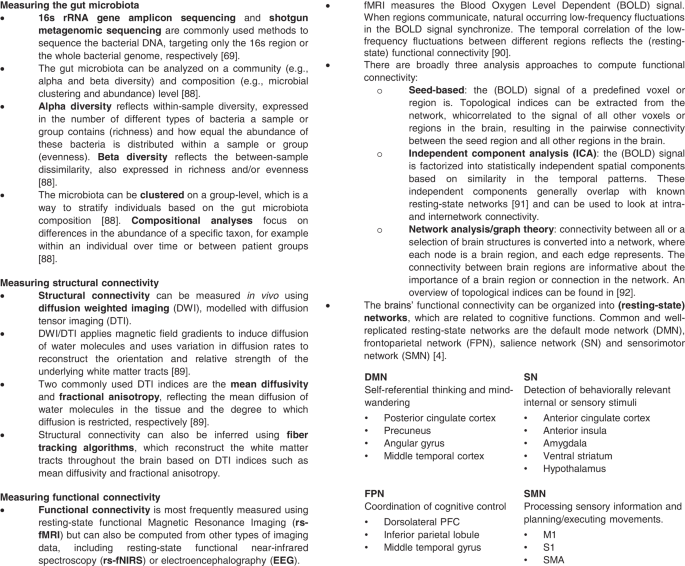
An overview of the methods and indices used to quantify and analyze the gut microbiota and functional and structural brain connectivity is provided in Fig. 2.
Microbiota quantification
The included studies employed various sequencing workflows to estimate the gut microbiota composition. Three studies performed shotgun metagenomic sequencing [36,